Configurations of ions
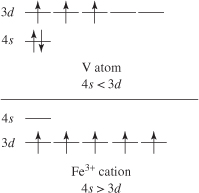
Writing the electronic configuration of ions follows the same rules as for neutral atoms. A cation has one fewer electron than the neutral atom for each positive charge and an anion has one additional electron than the neutral atom for each negative charge. So the electronic configuration of Na+ is 1s22s22p6 or [Ne] and that of F– is 1s22s22p6 or [Ne]. Na+ and F–are isoelectronic – these ions have the same total number of electrons (10).
Electron configurations of atoms and ions in conjunction with the location of the parent elements in the Periodic Table are of crucial importance in understanding the physical and chemical properties of the elements and their compounds.