Worked example 1
Draw the Lewis structure of carbon tetrachloride.
CCl4: C has the electron configuration [He]2s22p2 and that of Cl is [Ne]3s23p5, so C has 4 valence electrons and Cl has 7 valence electrons. The molecular framework of CCl4 is:
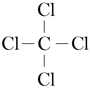
Addition of non-bonding pairs gives:
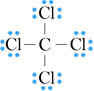
Note that in this structure, all atoms have formal charges of zero, and therefore there is no need to minimise formal charges on carbon and chlorine.