Worked example 3
Draw the Lewis structures of SO42−.
SO42−: Sulfur and oxygen both have 6 valence electrons. The framework of the molecule for sulfate ion is:
Addition of non-bonding pairs and accounting for a net charge on the sulfate ion of 2– gives:
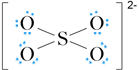
The formal charge on S is (6 – 4) = 2+ and that on each O is (6 – 7) = –1. The formal charge on S can be reduced to zero by converting one lone pair on each of two O atoms to bond pairs to give:
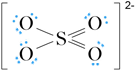
The formal charges on all atoms are minimised. Note that resonance structures can be drawn for this ion (see Worked example 5.2 in the textbook).