Electron affinity
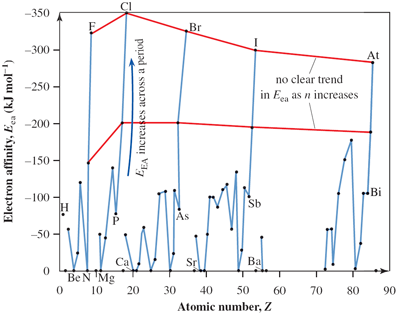
Electron affinity (EEA) is opposite to ionisation energy (Ei) and is the energy released when an atom gains one electron. For example, the electron affinity for F is –322 kJ mol–1 and relates to the process:
F(g) + e– → F– (g).
Electron affinity increases across a period. For example, EEA of P is –72 kJ mol–1, S is –200 kJ mol–1, Cl is –349 kJ mol–1, respectively.
Ionisation energies and electron affinities are both a measure of the tendency of atoms to form ions and hence are integral to understanding the characteristics and chemical properties of ionic compounds. The periodicity of Ei and EEA are particularly important in differentiating between ionic and covalent compounds and for rationalising chemical and physical properties.