Worked example 3
The compound C7H8O has infrared absorption bands over the range: 690–740, 1450–1490, 1700–2000 and 3310 cm−1. Based on correlation tables, what are the probable structures for this compound?
First calculate the IHD – reference hydrocarbon is C7H16
so IHD = (16 − 8)/2 = 4. The compound contains a benzene ring.
From correlation tables:
Absorption 690–740 cm−1 corresponds to aromatic C–H
Absorption 1450–1490 cm−1 corresponds to a benzene ring
Absorption 1700–2000 cm−1 corresponds to a benzene ring
Absorption 3310 cm−1 corresponds to –OH functional group.
- 4 structures are possible:
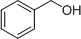
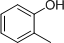
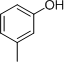
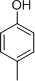
(Note: It is difficult to differentiate between these structures based on IR data alone.)