NMR spectroscopy and nuclear spin
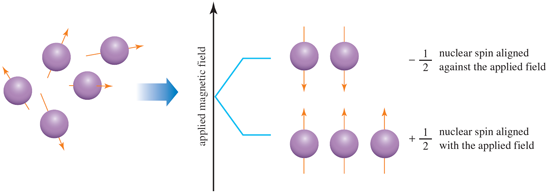
Nuclear magnetic resonance (NMR) spectroscopy gives information about the number and type of atoms in a molecule (Hydrogen: 1H NMR, Carbon:13C NMR ). Three concepts are crucial for understanding NMR: nuclear spin, shielding and chemical shift.
An atomic nucleus that has an odd mass number or an odd atomic number has a nuclear spin and behaves as a magnet in an (external) magnetic field. Thus, 1H and 13C have a nuclear spin but 12C does not. Interactions between nuclear spins and applied magnetic fields are quantised and only two orientations are allowed (aligned ‘with’ and aligned ‘against’ the applied magnetic field). Application of radio frequency radiation causes ‘resonance’ between the lower and upper energy spin states and a signal appears in the NMR spectrum.