Shielding
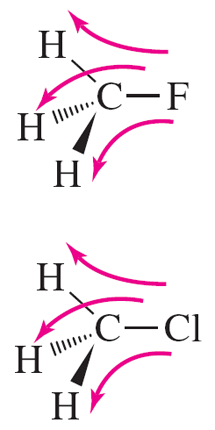
The electrons surrounding a nucleus also have spin and create ‘local’ magnetic fields which oppose the applied field thereby ‘shielding’ nuclei from the applied magnetic field. The greater the shielding effect, the greater is the applied field required to effect resonance. Electron density around a nucleus is influenced by the atoms that surround that nucleus. Thus, the electron density around the H atoms in fluoromethane is less than that around the H atoms in chloromethane, due to the greater electronegativity of F compared to that of Cl. Therefore the H atoms in chloromethane are more shielded than the H atoms in fluoromethane – or the H atoms in fluoromethane are more ‘de-shielded’ than the H atoms in chloromethane.