Equivalent hydrogen atoms and signal areas
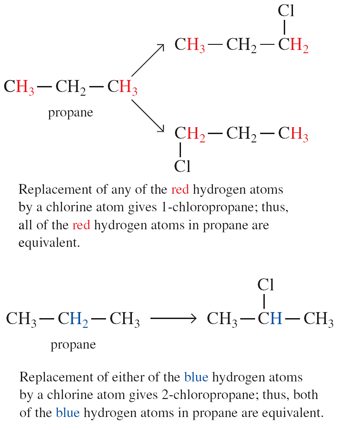
Equivalent hydrogen atoms give the same 1H NMR signals at the same δ value. Non-equivalent H atoms give different 1H NMR signals at different δ values. To decide which H atoms in a molecule are equivalent, each in turn is replaced by a ‘test atom’, such as halogen. If the replacement of two H atoms in turn gives the same compound, these two H atoms are equivalent; whereas, if replacement gives different compounds, the two H atoms are non-equivalent.
Integrated NMR spectra reveal the number of H atoms giving rise to each NMR signal, which corresponds to the number of equivalent H atoms giving rise to a particular δ value.