Ionic radii
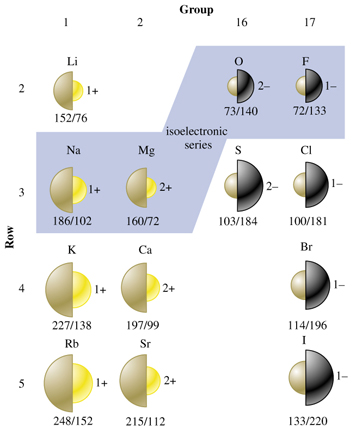
Cations are smaller and anions are larger than the parent atom due to the effective nuclear charge being greater for cations and less for anions as compared to the parent atom. For example, the ionic radius of Li+ is 76 pm compared to that of Li, 152 pm, and the ionic radius of Cl– is 181 pm compared to 100 pm for the Cl atom. For the same charge, cation and anion radii increase down a group. For example, the ionic radius of Na+ is 102 pm, Rb+ is 153 pm, Cl– is 181 pm and I– is 220 pm. The ionic radius trend across a period is only apparent for isoelectronic species. For example, O2– is 140 pm, F– is 133 pm, Na+ is 102 pm and Mg2+ is 72 pm. So for isoelectronic species, ionic radii decrease across a period.