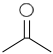
Worked example 2
C3H6O gives only one signal in its 1H NMR spectrum. Propose a structure for this compound.
First determine the IHD of C3H6O; the corresponding saturated hydrocarbon is C3H8
so IHD = (8 − 6)/2 = 1, so there is one (C=O) double bond present.
Only 1 1H NMR signal, so all 6 H atoms are equivalent and hence the structure is: